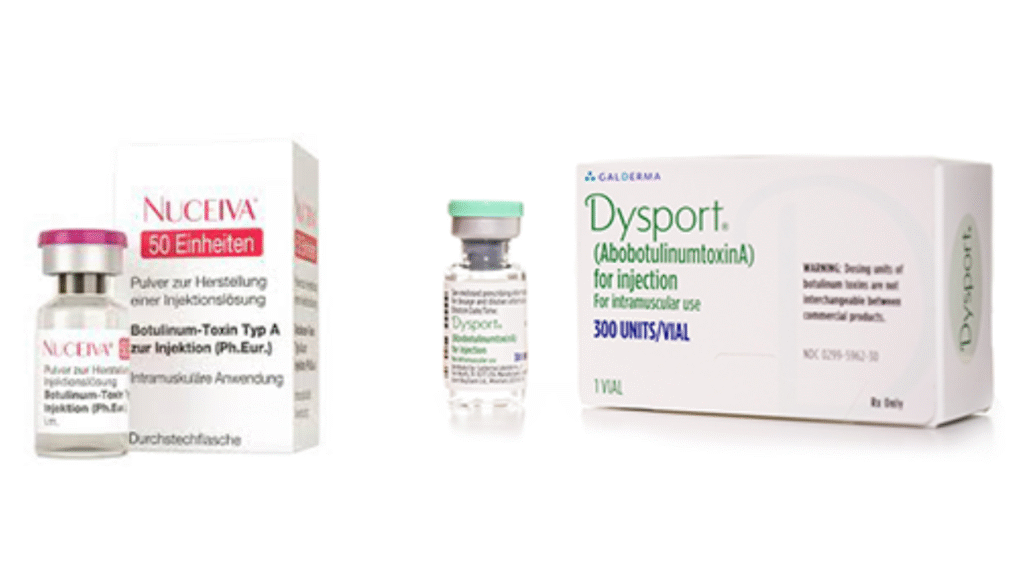
On Oct 16, 2019 Clarion Medical Technologies, a leading Canadian provider of medical and aesthetic equipment and technologies, announced the first shipment of the “Newtox” Nuceiva (prabobotulinumtoxinA) in Canada. Nuceiva is a 900 kDa purified botulinum toxin type A formulation that was authorized for sale by Health Canada in August 2018 for treating moderate to severe glabellar lines in adults. Clarion is the only authorized provider of Nuceiva in Canada and is exclusively partnered with Evolus, an American company based in Newport Beach, California. The product was approved by the US Food and Drug Administration in February 2019 and launched in the US by Evolus under the brand name Jeuveau in May 2019.
The “Newtox” Nuceiva is similar to other botulinumtoxinA products. It is a 150 kDa toxin within a 900 kDa protein complex, like Botox. Each vial contains 100 units of the neurotoxin complex, 0.5 mg of human serum albumin and 0.9 mg of sodium chloride. It is preservative free.
Battle to launch Jeuveau/Nuceiva in the US
It is interesting to note that there is a battle going on between Allergan along with its South Korean partner MedyTox Inc. (the makers of Botox) and Evolus and its South Korean partner Daewoong Pharmaceutical Co. (the makers of Jeuveau/Nuceiva). In December 2020, Allergan won a ruling that Jeuveau should be banned in the US as it is made using MedyTox’s secret process that turns the deadly botulinum toxin into a cosmetic product. In February 2021, at the request of Daewoong Pharmaceutical Co., the US Court of Appeals allowed Jeuveau to remain for sale in the US temporarily while it considers staying the import ban.
Nuceiva vs Dysport: an Experiment
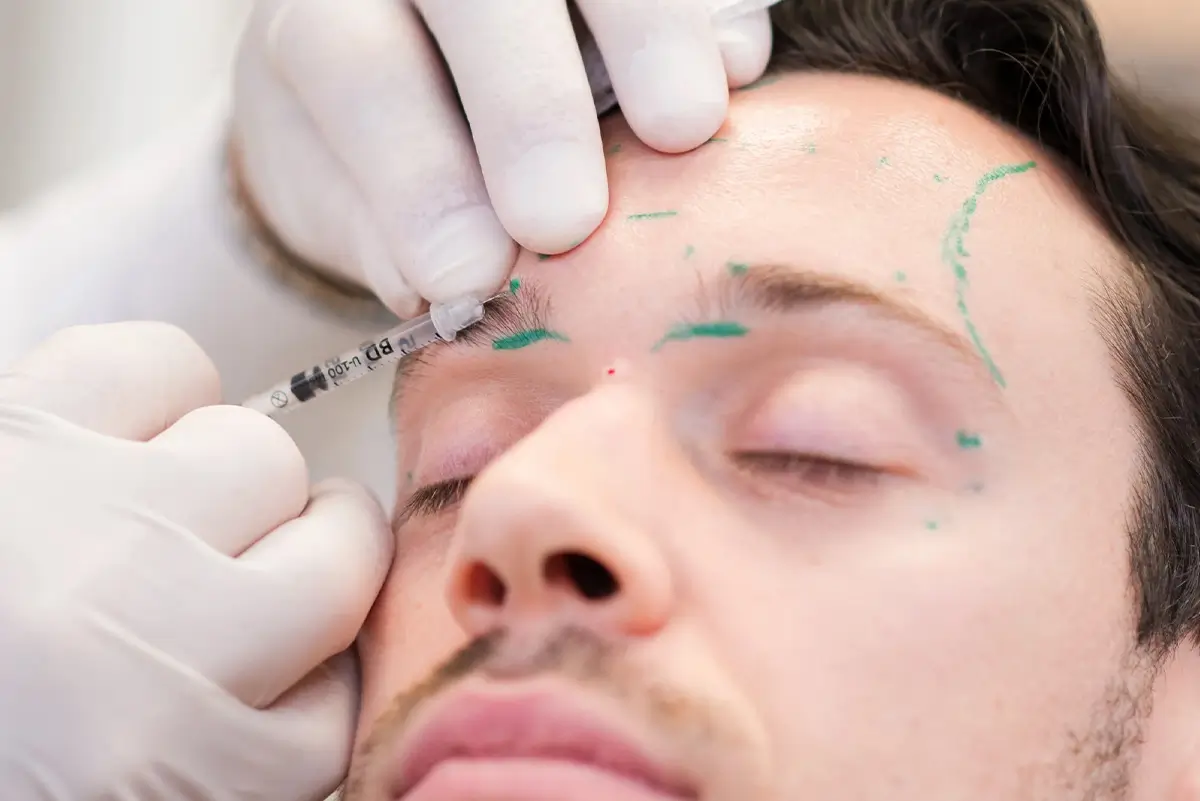
In January 2021, PTIFAT undertook a small experiment to compare the results of injections of Nuceiva versus Dysport (another botulinumtoxinA) in the upper face. Dr Warren Roberts was the subject for the comparison and Dr. Jan Roberts was the injector. The two products were reconstituted the same way (1 ml of 0.9 mg preserved saline to 1 vial). One product was injected on the right side of the face and the other product on the left side.
Treatment:
A total of 30 units was injected into frontalis, 2 units in each of 15 sites, and 46 units into 7 sites in the glabella. In the double-blind study, neither the injector nor the patient was aware which product was used on each side. Here are the marked photos of the patient’s treatment plan.
Post-Op Results:
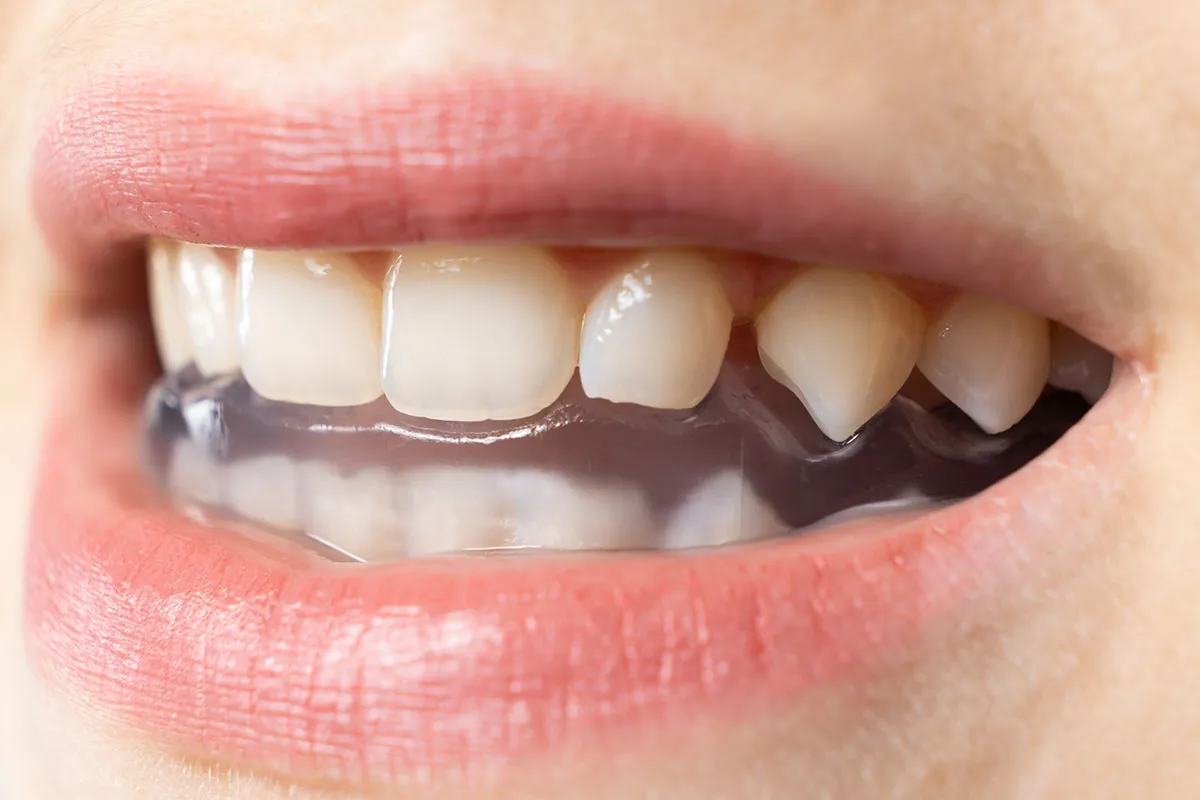
Before treatment photographs were obtained and then again two weeks post treatment (see below). Both products produced a reduction in both glabellar frown lines and forehead lines. The left side of the forehead exhibited fewer lines in activation than the right side, while both sides of the glabella appeared much the same. The photographs below illustrate the results.
Conclusion:
It was revealed after the final photos were obtained, that the right side had been injected with Nuceiva and the left with Dysport. It appears that there was a slightly greater improvement using Dysport over Nuceiva in this one clinical case. Drs. Roberts plan to perform similar experiments in the coming future. More case results will be shared in the online Study Club.